My ESPriT2 prediction
Lucy Letby – no more from me.
I had intended to post a mother-by-mother analysis of the publicly available obstetric details, to show how consideration of the pregnancies and births might have helped the court. But my scene-setting (here, here & here) led Letby’s new defence team to reach out.
It would be inappropriate to comment publicly, once I’ve seen the records, so I won’t post any more for now.
Jim Thornton
The parents were granted anonymity, so the court labelled the children chronologically as “Child A, B, … Q”. Here is the appeal court’s summary of the charges for which Letby was convicted in the first trial and the methods she used.

In addition there was child K, for whom the original jury were unable to agree a verdict. Letby was convicted at a second trial of the attempted murder of child K by dislodging the endotracheal tube.
She was also found not guilty of the attempted murder of baby H – dislodged ET tube, and the jury could not reach a verdict on baby J, attempted murder by airway obstruction, and on baby Q, attempted murder by air via the NG tube.
In summary Letby was convicted of using seven, or possibly eight, different methods to attack babies.
- Air embolus – seven times,
- Air via nasogastric tube – three times,
- Overfeeding with milk – twice, but both on the same baby,
- Insulin poisoning – twice.
- Throat trauma – once.
- Injury to liver – once.
- Dislodged endotracheal tube – once.
- “Gastric trauma using a rigid medical tool” – once. This last method is my interpretation, based on the BBC reports at the time (click here), of what the appeal court meant by “acute bleeding” for baby E. “Acute bleeding” seems more a mechanism of death, than a mechanism of murder.
Shoo Lee International Panel numbering
The Shoo Lee panel numbered the children 1 to 17, although they followed the court’s lettering order. They published their summaries in two parts (here and here), not in number order, together with much extraneous author biographical detail.
I’ve therefore prepared a merged Shoo Lee panel summary document with both numbers and letter codes (click here). For convenience I’ve removed the panel’s biographical details and re-ordered the cases in number/letter order, the same as the court. I have not altered the panel’s text.
Obstetricians should be aware of a further minor lettering and numbering issue. The court lettered the babies of multiple births in the order Letby was alleged to have attacked them, rather than in birth order. This is the reason baby 1/A was the second twin and baby 2/B the first. It also explains why triplet 15/O was born after triplet 16/P.
Staff anonymity
Nine of the doctors and nurses who worked alongside Letby were also granted anonymity, so although we know the identity of three consultants, Drs Jayaram, Breary and Gibbs, three others are known only as Drs B, ZA, and Dr V. One of the trainee paediatricians is known only as Dr A in the trial but confusingly as Dr U at the Thirwall inquiry. I’ve not attempted to sort out these staff name codes.
Neonatal unit levels
There are three levels of neonatal unit in the NHS (click here).
Special Care Unit (SCU), or Level 1
For babies who do not need a high level of care, and are born after 32 weeks’ gestation and where care is limited to monitoring, tube feeding, phototherapay and oxygen via face mask. Such units should also be capable of initial stabilisation and support for unwell or more preterm babies before transfer.
Local Neonatal Unit (LNU), or Level 2
For babies who need a higher level of care, born after 27 weeks’ gestation, and weighing over 1000 grams. Level 2 units provide short term intensive care including ventilation (up to 48 hours), non-invasive ventilation such as continuous positive airway pressure (CPAP), and parenteral nutrition.
Neonatal Intensive Care Unit (NICU), or Level 3
Everything else, including long term ventilation, feeding support, and cooling therapy to reduce brain injury. Some level 3 units also provide specialist neonatal sugery, ECMO or other highly specialised services.
All regions also have a neonatal transport service capable of collecting the sickest babies from level 1 and 2 units.
The Countess of Chester hospital
In 2015/16, the period of Letby’s crimes, the Countess of Chester was classed as a local neonatal, level 2, unit.
In July 2016, when Letby was removed from direct clinical duties, never to return, it was downgraded to a special care, level 1 unit.
That’s enough background. Tomorrow I’ll make a start, taking each mother in turn. First the mother of twins 1/A & 2/B (click here).
Update 19 June. There’s been a change of plan (click here).
Jim Thornton
Lucy Letby – obstetric intro & summary
As far as I know, no obstetrician appeared as an expert witness at the trial. This may not surprise most people; the allegations were of murder and attempted murder in a neonatal unit. But it’s surprising to obstetricians, because almost all neonatal problems have their origin in pregnancy. Prematurity obviously, but much else as well.
It is therefore even more surprising that no independent obstetrician appears to have even looked at the mothers’ clinical notes.
The evidence that this may be so comes from Professor Neena Modi, a member of Shoo Lee’s expert panel. She says the panel had asked for, but not been given, the mothers’ notes. For the full interview (click here). For 16.33 where she makes this point (click here).
Professor Modi is not just any professor. When the offences took place, she was the President of the Royal College of Paediatrics and Child Health, arguably the most senior paediatrician the UK. She is a precise and careful witness.
Since the panel had all the notes available to Letby’s new legal team, and the new legal team had been given all the notes available to her old legal team, and the prosecution has a duty to pass all their evidence to the defence, it seems reasonable to infer that neither side had copies of the mother’s records. If they did not have them, they cannot have looked at them.
Of course much information will have been copied into the baby notes, and the route of birth, and whether the baby was a multiple, is obvious. Growth restriction and fetal distress can also be inferred from the baby’s weight and condition at birth. And the parents will remember a lot.
But the significance of some details may not have been appreciated at the time. A transcribing nurse or doctor can hardly have imagined that years later a court would have to decide if a baby had died from natural causes or been murdered. The details may matter.
To take just a simple example. Consider an imaginary preterm baby, say 29 weeks, born by Caesarean because the mother had raised blood pressure, who later collapsed and died.
The baby notes will likely record the gestation, the Caesarean and the blood pressure. But they might miss the exact BP level, how long had it been raised, any response to treatment, whether the mother also had pre-eclampsia, or other complications, and the extent to which the blood pressure was compromising the baby’s health?
The baby notes will likely record whether the mother was in preterm labour or not, and if not, why the obstetricians had decided to deliver then. But they will rarely record all the maternal, fetal and other factors that had led to the choice of that precise gestation.
The baby notes will probably also record the main steps taken to reduce the risks of prematurity, whether steroids and magnesium sulphate were given, and whether clamping of the umbilical cord was delayed. But they might miss the precise timings. They should record obvious surgical difficulties but might miss the details. They rarely record the seniority of the surgeon.
The baby notes usually record whether the mother was given a spinal or general anaesthetic but typically little more about the anaesthetic.
The Shoo Lee panel have also alleged that poor, and occasionally even negligent, care was a factor in some deaths, and that the neonatal unit staff were neither qualified nor experienced enough to deal with some of the sickest babies.
If this may apply to the neonatal unit at the the Countess of Chester Hospital, it may also apply to the maternity unit. Only by reading the mother’s notes can we be sure that the pregnancy was managed correctly. The baby notes will rarely contain sufficient information to judge that.
Of course even if different obstetric care might have altered the outcome, that doesn’t necessarily imply negligence. There’s a wide area for expert judgment, and it’s easy to criticise with the benefit of hindsight. But it might be relevant to whether death was by natural causes or murder.
Anyway here’s what the Shoo Lee panel found in the baby notes. Sources here and here. Merged into trial order with both numbers and letters here. Many babies had more than one issue.
- Three full sets of twins (six babies), two of a set of triplets, plus two other individual twins from separate sets (two babies). In total ten babies came from a multiple pregnancy. The twin complications included at least two cases of twin to twin transfusion syndrome (TTTS), one so severe that it had required laser ablation of the placental vessels connecting the twins’ circulations.
- All but one baby was preterm (<37 weeks). Three were extremely preterm (<28 weeks), six very preterm (29-32 weeks) and six moderately preterm (33-36+6 weeks). Six babies also suffered from intra-uterine growth restriction.
- Thirteen babies were born by Caesarean, two vaginally, and for two the route was not noted by the panel.
- There were also cases of chorioamnionitis, pre-labour membrane rupture, a maternal haemophilia carrier, obstetric cholestasis, birth in a toilet, footling breech birth, one mother with placenta praevia/accreta, one with diabetes, and one with hypertension & antiphospholipid syndrome.
Plenty for an obstetrician to think about.
But first I need to sort out the babies’ letter and number codes, and explain about the different levels of neonatal unit (click here).
Jim Thornton
Lucy Letby – an obstetric view
Background
I am emeritus professor of obstetrics & gynaecology at the University of Nottingham and, until I retired in 2024, was a consultant obstetrician at Nottingham University Hospitals NHS Trust. www.ripe-tomato.org is my blog
A year ago I read A British Nurse Was Found Guilty of Killing Seven Babies. Did She Do It? by Rachel Aviv in the New Yorker (click here). The baby K retrial was ongoing, and the courts had blocked the UK online version, but I had a paper copy.
Aviv argued that no-one witnessed Letby attacking any baby, and that the apparently incriminating notes containing the words, “I am evil”, “I killed them”, were neither a confession nor incriminating, when read in their entirity.
There had been a spike in deaths, but the Countess of Chester hospital had other problems, and the mortality fall after she was removed from clinical duties, coincided with the hospital ceasing to care for the sickest babies.
Letby seemed to have been convicted largely on the basis of being the only person present when all the suspicious events occurred, but statisticians Aviv spoke to, suspected that the events had been selected because she was present, the so called Texas Sharpshooter fallacy.
The main prosecution expert had offered his services to the police, had little up-to-date expertise in neonatology, and seemed more keen on helping get a conviction, than as acting as an independent advisor to the court. His theory that some deaths had been caused by air embolism was based on a misunderstanding of the paper he cited.
When the police were alerted to the possibility that there might be a multiple murderer active in the neonatal unit, they had allowed the accusing medical staff, against whom Lucy had recently had a claim of bullying upheld, to lead the search for evidence, rather than including them in the list of suspects.
The two insulin cases, which the prosecution argued proved that there was a poisoner in the unit, were based on a tricky insulin/c-peptide assay done by a non-accredited lab. The results had been ignored at the time, and the babies recovered, so they couldn’t be rechecked. Letby was not charged for a third insulin case of which the defence was unaware. Aviv argued that these weak insulin cases dragged the rest behind them, in the “belief that all the deaths couldn’t have occurred by chance”.
I’m not a true crime buff, nor a miscarriage of justice campaigner, but the article disturbed me. The New Yorker doesn’t usually get stuff wrong; it’s rather famed for its obsessive fact checking. Since then more information supporting Aviv’s concerns has come to light.
The incriminating notes may have been written on the advice of a counsellor while Letby was suspended for alleged incompetence and felt bullied.
Professor David Spiegelhalter has explained to the Thirwall inquiry that the spike in deaths was not outside what might easily arise in a relatively poorly run unit (click here).
We now know, what the statisticians had suspected, that many events and deaths had first been labelled suspicious on the basis of Letby’s presence, and that others were discarded if she was absent (click here).
Hardly a month goes by without new problems appearing around the insulin evidence.
In the only case (Baby K) where Letby was witnessed doing anything, namely standing doing nothing over a desaturating baby, the witness, Dr Jayaram, had made no note at the time, not even privately to his colleagues, despite them all already suspecting she was a murderer. Instead an email has recently come to light (click here) in which he had written, apparently contradicting his later sworn testimony, that Letby had called him into Baby K’s room.
But far more important than any of that, in February this year, an international expert panel, convened by Professor Shoo Lee, the expert whose research had been misunderstood, identified straightforward non-malicious explanations for all the deaths and collapses. “We did not find any murders”. Click here.
I’m not going to go over all the Shoo Lee panel’s arguments here, but the air embolism misunderstanding is not complicated.
Air accidentally entering a pulmonary vein, the topic of the misquoted Lee and Tanswell paper (click here), passes into the systemic circulation and, when it reaches the skin capillaries, may cause a specific skin rash. In contrast air injected into a peripheral vein1, the act Letby was accused of, has to first pass through the lung capillaries where it will be trapped. Anyone who knows the difference between an artery and a vein, and has got their head around William Harvey’s discovery of the blood circulation, can understand the difference.
Shoo Lee himself caused a potential confusion by mentioning the theoretical possibility that injected peripheral vein air could pass through the foramen ovale, a normal hole in the heart to reach the systemic circulation and the skin. He dismissed this possibility in practice, because the foramen ovale closes at birth, and even in the rare cases where it does not, the pressures on the different sides of the heart after birth would prevent air bubbles flowing in that direction. Every obstetrician and neonatologist knows all about the transition from the fetal to the adult circulation at birth, so knows that Shoo Lee is correct. Finally, to remove any last doubt, Shoo Lee wrote another paper last year looking specifically for reports of patchy skin discoloration in infants with peripheral venous air embolism (click here). There have been zero such reports.
But none of this new evidence has been tested in court. Letby has already been refused two appeals and will spend the rest of her life in prison unless the Criminal Cases Review Commission (CCRC), decides to take her case on.
In the meantime, although almost all doctors I’ve discussed the case with have serious doubts, relatively few have come out publicly in Letby’s defence.
The reason is probably that few working doctors have time to read all the details, and it’s risky publicly defending a convicted child killer. One of the few practicing neonatologists to speak out has had people complain about her to the GMC and to her employer.
I retired last year, so I’ve got the time and don’t really care if people write to the GMC about me. But I’m not a neonatologist, nor even a paediatrician, so what can I add?
I was an NHS obstetrician for over 40 years, and in clinical practice in 2015/16, when the events unfolded. Since no obstetrician appears to have commented on the case in detail, I’m going to try and fill that gap, and focus on the maternal aspects, the obstetrics.
My plan is to collect what information I can about the pregnancies of each mother, and provide a brief obstetric commentary. Since I’ve not got access either to the mother’s notes, nor to the trial transcipt, I will rarely be able to come to a firm conclusion. But I may be able to point to useful lines of enquiry.
For each pregnancy and birth I will look for any maternal disease, or potentially substandard obstetric care, that might have contributed to the babies’ later deaths or collapses. If further information comes to light, I will update.
I will also consider whether it had been appropriate at the time for each mother to have delivered in The Countess of Chester, a level 2 unit, and if not, whether opportunities were missed for transfer to a level 3 unit.
Finally I will consider whether it would hypothetically have been appropriate to deliver the mother in a level 1 unit, the level to which Chester was downgraded in July 2016. This may be relevant to the fall in the baby deaths at Chester at that time.
Tomorrow I will explain why this task may be relevant, and why I believe it has not already been done either by the prosecution or defence. I will also summarise the maternal information the Shoo Lee panel found in the baby records (click here).
Jim Thornton
Edited, 5 June, to clarify that Letby was “refused” rather than “lost” two appeals. Edited, 9 June, to clarify that air cannot be injected into a pulmonary vein. It may “accidentally enter” a pulmonary vein.
- Footnote added 3 October 2025.
People have told me that Letby was convicted of injecting air into an already installed intravenous line, or an intravenous long line, rather than cannulating a peripheral vein herself, and asked whether either possibility would invalidate the Shoo Lee panel’s hypothesis.
It is correct that the prosecution alleged that she had injected air into a pre-existing line. It would be difficult to freshly cannulate a newborn baby’s peripheral vein without being noticed.
However, air into peripheral veins or long lines are the same thing in this context. A long line passes up the peripheral veins, so the air would enter the bloodstream nearer the heart. But it would still arrive at the right side of the heart and need to pass through the lung arteries and into the lung capillaries, where it would be trapped, before it could return to the the left side of the heart to be pumped out into the systemic ciculation and appear in the skin. ↩︎
mPox in pregnancy. Totals of published cases.
Here we, mainly Keelin O’Donoghue, make our best guesses about duplicates. Click here for links to primary sources.
Pre 2020 (Clade 1)
Total 8 reported cases. 5 Congo (MP1, MP6, MP21, MP22, MP27). 1 Zaire (MP3, MP29, MP30). 2 Nigeria (MP2, MP25). Note no pregnancy cases in the 2003 USA outbreak (MP4)
2020 onwards
Total 38 reported cases. 9 Congo (MP28). 27 USA (MP12, MP17, MP18, MP19, MP31). 1 UK (MP14). I Spain (MP20).
These 38 are likely included in 60 collected by WHO (MP23, MP26)
mPox in pregnancy. News reports
For scientific primary sources for mPox in pregnancy (click here). In this regularly updated post we list mainstream news reports only. As far a possible we avoid duplicate reports. To date, August 2024, in contrast to Covid-19 (click here), there have been no celebrity cases of mPox in pregnancy.
August 2022
An unnamed 37-year-old pregnant woman in Campinas, Sao Paolo state, Brazil, contracted mPox. The pregnancy is ongoing (click here). Report 5 August 2022
July 2022
An unnamed woman in the US contracted mPox during pregnancy (click here). Both survived. The baby was given prohylactic IgG. The many news reports of this case probably refer to MP studies 12 and 17 (click here). Report 26 July 2022
November 2017
An unnamed woman from the Congo with suspected mPox (tests awaited) miscarried at 16 weeks (click here) Report 9 November, 2017
River Main; from Bamberg to the Rhine
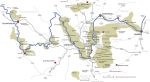
Flowing east to west for nearly 400 km, I’ve divided the Bamberg to Rhine section into four; Bamberg to Schweinfurt (brewery section), the triangle (Maindreieck), the rectangle (Mainviereck) and finally Frankfurt and the Rheingau. All distances in km from the Rhine junction (click here). There are plenty of official campsites, and although wild camping is verboten in Germany, many tempting spots. One local told me that the authorities turn a blind eye to a single night, but sometimes get forced to act by “busybody Bavarians” – his words! Another that the law defines “camping” as having a groundsheet under you. I was lucky with the weather, pulled up late and laid my bag directly on the ground. No-one complained.
1. Bamberg to Schweinfurt – the brewery section
The Main-Donau canal is not recommended. Although Bamberg is the obvious start, and camping Insel (click here) a lovely site on the Regnitz, about 5 km upstream of the junction, there’s no easy route through Bamberg. The Regnitz right fork, leading to the Main-Donau canal, with little flow in summer, is blocked by a barrage, albeit a possible portage.



The left fork through the hydroelectric power plant in the centre of the Old Town is scenic, but… .






Bamberg old lock is not functioning, but could be portaged via the ferry landing,



The real problem is 6km downstream at Viereth. No sport boat lock and no access. The least bad option would be to land right above the motorboat club and portage nearly a kilometre round the old river loop. Not for me.




Fortunately all other weirs have a sport boat lock, or an easy portage, or both, so I started below Viereth. This upper section is famous for its beer brewing. There are allegedly five breweries in Bamburg alone (click here), and even the smallest town downstream usually has its own.
380.5 km -Viereth lock. Depth 6m. Hydroelectric power station right. 6MW. Launch right bank upstream of the S12262 bridge. Viereth left. Memorial to Michael translates roughly as “Far from the eye… Forever in the heart.”




371 km – Eschenbach left. Home to the Eschenbach brewery (click here).



Stettfeld, on the opposite bank, albeit not easily accessible behind gravel pits and the E48 motorway, has the tiny Adler-Brau brewery and bar (click here).



369 km – B26 bridge. Motor boat club, marina, and campsite left 100m before the bridge. Eltmann left. Ebelsbach right. Eltmann’s brewery is Franz Engel. No website but they host a beer festival in July. The Laurenz Krug brewery in Ebelsbach ceased operations in 1991; even Germany has experienced some consolidation. Looks like there was a ferry once.






367.5 km – A70 bridge Followed by 367 km – Limbach lock. Limbach left. Volland brewery closed in 1971. The sport boat lock to the right of the island opens into the original river meander.




363.5 km – Sanderstrasse bridge. Sand left. Zeil, followed by Zeil wharf, right.



0.5 km after the bridge enter the lake via a gap in left bank. The arrow on the sign is ambiguous, but this is the route to Camping am See (click here) at the far end of the lake. Lakeside pitches are reserved for long stay visitors. Overnighters camp near the reception office, 120 metres up the road. There also appear to be plenty of wild camping spots nearer the river.





We are now at the absolute limits of the Franken wine region. The Sander Kronberg vineyard lies in woodland south of the town, well away from the river. Grapes have been grown in Zeil for a thousand years and today there are still a few small vineyards on the south facing “Ziegelander” slopes behind the town. There are also three wineries in Zeil, Martins Klause (click here), Nusslein (click here) and Dr Heigel (click here). Three or four in Sand, Bernhard Rippstein (click here), Thomas Schutz (click here). The day I visited there was a Weinfest, although it looked more like a music festival with crowds, queues and bands.


361.5 km – ST2427 bridge, followed by 360 km – Knetzgau lock right. 4.24 M deep. Hydroelectric power station left 2.9MW.
Lock operation is easy. Talfarht means downstream and Bergfarht upstream. Push the handle to the direction you want the lock to work and press the big button. For safety the big button has to be held down to work the gates. And unnervingly, for those like me who are unsure that they’ve got everything right, there is often a substantial delay before the gate starts moving. If you’re working the lock singlehanded, you’ll need to sit in the canoe while the lock empties, tie it up, climb up the ladder to open the lower gate, and finally climb back down to paddle off. Scary the first time.






356 km – Camping right bank at Hassfurt (click here). Or land at Hassfurth harbour, followed by 355 km – Hassfurt bridge. The Hassfurt Henneberg vineyard is in the village of Prappach a couple of km to the north east. See Weingut Eller (click here).




354 km – Nassach stream joins right.

349 km – Obertheres motor boat club right. Overnight camping (click here) on the cycle path. Excellent spot. Obertheres right. Followed by 348 km – St2426 bridge



345 km 345 km – Ottendorf lock right. 7.6M deep. Hydroelectic power station left 6.5 MW





344 km – footbridge. Untereuerheim left. Gadheim right. Swallows nesting in the sandbanks




342 km – B303 bridge

338 km – Ferry site, or ford, or Nato ramp. Lidl right. 337 km – Schonungen right. Steinach stream enters right.


335 km Google maps mark Kanu Anlegestelle Mainberg in front of Schloss Mainberg. A good launch spot, but too busy for a wild camp.



No campsite in Schweinfurt. I bivouacked by one of the distance markers, a few hundred metres before the town.
332.5 km – Max Bridge. Schweinfurt right. 332 km – Schweinfurt big lock left, Island, Hydroelectric power station 3.8MW, weir, Small boat lock right. 4.7M deep. The industrial sculpture on right bank commemorates the world’s first roller dam, installed in the channel at the left end of Max bridge in 1902. I think all the modern dams on the Main are also roller dams.





Turning south at Schweinfurt the river leaves beer country, and enters the Maindreieck or triangle. (Click here for River Main – 2).
Larkin in a thousand words
My first ChatGPT
I entered “The life and works of the poet Philip Larkin in 1000 words” here. 450 came back.
Philip Larkin (1922-1985) was a British poet, novelist, and librarian who is widely regarded as one of the most important voices in post-World War II British poetry. Larkin was born in Coventry, England, and attended Oxford University, where he studied English literature. After graduating, he worked as a librarian at the University of Leicester, the University of Belfast, and finally the University of Hull, where he spent the majority of his career. Larkin's poetry is known for its bleak, often pessimistic tone, and its focus on the mundane aspects of everyday life. He is often associated with the movement known as "The Movement," a group of poets who rejected the avant-garde experimentation of their predecessors in favor of a more traditional, accessible style of poetry. One of Larkin's most famous poems is "This Be The Verse," which begins with the lines "They fuck you up, your mum and dad / They may not mean to, but they do." The poem's frank, unsentimental tone and its focus on the damaging effects of family relationships made it a controversial work when it was first published in 1971. Another notable work by Larkin is his collection "The Whitsun Weddings," which includes the poem of the same name. The collection explores themes of time, mortality, and the passage of seasons, and is widely regarded as one of Larkin's best works. "The Whitsun Weddings" is also notable for its use of vernacular language and its depiction of working-class life in post-war Britain. Larkin's other notable collections include "The Less Deceived," "High Windows," and "Collected Poems." His poetry is characterized by its clarity, its precise and unadorned language, and its ability to find beauty in the mundane. In addition to his poetry, Larkin was also a respected novelist and essayist. His novel "A Girl in Winter" (1947) is a bleak and haunting portrayal of life in wartime England, and his essays, collected in "Required Writing" and "Further Requirements," are celebrated for their wit and insight into the human condition. Despite his literary achievements, Larkin was also known for his reclusive personality and his controversial views on race and politics. In his private life, Larkin was a heavy smoker and drinker, and he never married or had children. He died of cancer in 1985, and his funeral was attended by a small group of close friends and colleagues. In the years since his death, Larkin's reputation has continued to grow, and he is now widely regarded as one of the most important British poets of the 20th century. His influence can be seen in the work of countless poets who have followed in his footsteps, and his legacy continues to inspire new generations of readers and writers.
No Kingsley Amis, Monica Jones or jazz. But not bad. “The movement known as ‘The Movement'” could be rephrased. And surely the F word, rather than the unsentimental tone, made TBTV controversial. But these are quibbles. Let’s try again.
Philip Larkin was a British poet, novelist, and librarian, known for his simple yet powerful language, and for his explorations of themes such as death, love, and the passage of time. Born in Coventry, England in 1922, Larkin was educated at the University of Oxford, where he studied English literature. He went on to work as a librarian for many years, first at the University of Leicester, and later at the University of Hull, where he served as the head librarian from 1955 until his retirement in 1985. Larkin's poetry is often characterized by its stark realism and its unsentimental portrayal of everyday life. His most famous poem, "This Be The Verse," begins with the line "They fuck you up, your mum and dad," and goes on to explore the idea that our childhood experiences can have a profound and lasting impact on our lives. Larkin's work is often bleak and pessimistic, but it is also infused with a sense of dark humor and irony, as he skewers the pretensions and hypocrisies of modern society. One of Larkin's most famous collections of poetry is "The Whitsun Weddings," published in 1964. The title poem describes a train journey from Hull to London on the day of the Whitsun weddings, and it is widely regarded as one of Larkin's finest achievements. The poem captures the sense of excitement and anticipation that pervades the train as it speeds towards the capital, but it also explores the darker themes of mortality and the passing of time. Another of Larkin's most famous poems is "An Arundel Tomb," which describes a medieval tomb in a church in Sussex. The poem reflects on the nature of love and the transience of human life, as Larkin observes that the effigies of the tomb's occupants have been "preserved just so by what they did / And if you look long enough / They will begin to move." The poem has been interpreted as a tribute to Larkin's own parents, who are buried in the same churchyard. Larkin's novels include "Jill" (1946), "A Girl in Winter" (1947), and "The Less Deceived" (1955). Although he is primarily known as a poet, Larkin's prose is also marked by its clarity and economy of expression. His novels often explore the themes of loneliness and isolation, and they are notable for their vivid characterizations and acute observations of human behavior. Despite his reputation as a master of gloomy verse, Larkin was also capable of writing lighter and more playful poetry. His poem "Vers de Société," for example, is a witty and urbane satire on the social mores of the middle classes. Similarly, his poem "High Windows" is a bawdy and irreverent celebration of sexual liberation, in which Larkin declares that "we should be careful / Of each other, we should be kind / While there is still time." Larkin's life was marked by a certain amount of personal tragedy. He never married and had few close friends, and he suffered from depression and anxiety throughout much of his life. He was also deeply affected by the death of his mother, who passed away when he was just 25 years old. Despite these difficulties, however, Larkin continued to produce some of the most important and enduring poetry of the 20th century, and his work remains widely read and celebrated to this day. Philip Larkin died in 1985 at the age of 63, but his legacy lives on in the countless readers and writers who have been inspired by his poetry. His influence can be seen in the work of many contemporary poets, who have taken up his mantle of stark realism and
Less good. He was 55, not 25, when his mother died. “We should be careful…” is from The Mower not High Windows. The Less Deceived wasn’t a novel. An Arundel Tomb is not about his parents, and they weren’t buried in the churchyard.
Humans can still beat AI, just.
Jim Thornton
The Skip
by James Fenton
A friend told me today that this poem had recently cheered up one of his elderly relatives while she recovered from a brush with the orthopods.
I confess I wasn’t familiar with Fenton, but he was friends with Martin Amis and Christopher Hitchens. This photo by Amis’s then girlfriend Angela Gorgas, was taken around the time the poem was written.

That’s good enough for this Amis and Hitchens fan, so I read the poem to my friend. It cheered us both up.
Jim Thornton
The Skip, by James Fenton
I took my life and threw it on the skip,
Reckoning the next-door neighbours wouldn’t mind
If my life hitched a lift to the council tip
With their dry rot and rubble. What you find
With skips is – the whole community joins in.
Old mattresses appear, doors kind of drift
Along with all that won’t fit in the bin
And what the bin-men can’t be fished to shift.
I threw away my life, and there it lay
And grew quite sodden. `What a dreadful shame,’
Clucked some old bag and sucked her teeth: ‘The way
The young these days … no values … me, I blame…’
But I blamed no one. Quality control
Had loused it up, and that was that.
‘Nough said. I couldn’t stick at home. I took a stroll
And passed the skip, and left my life for dead.
Without my life, the beer was just as foul,
The landlord still as filthy as his wife,
The chicken in the basket was an owl,
And no one said: `Ee, Jim-lad, whur’s thee life?’
Well, I got back that night the worse for wear,
But still just capable of single vision ;
Looked in the skip; my life – it wasn’t there!
Some bugger’d nicked it – without my permission.
Okay, so I got angry and began
To shout, and woke the street. Okay. Okay!
And I was sick all down the neighbour’s van.
And I disgraced myself on the par-kay.
And then … you know how if you’ve had a few
You’ll wake at dawn, all healthy, like sea breezes,
Raring to go, and thinking: `Clever you!
You’ve got away with it.’ And then, oh Jesus,
It hits you. Well, that morning, just at six
I woke, got up and looked down at the skip.
There lay my life, still sodden, on the bricks;
There lay my poor old life, arse over tip.
Or was it mine? Still dressed, I went downstairs
And took a long cool look. The truth was dawning.
Someone had just exchanged my life for theirs.
Poor fool, I thought – I should have left a warning.
Some bastard saw my life and thought it nicer
Than what he had. Yet what he’d had seemed fine.
He’d never caught his fingers in the slicer
The way I’d managed in that life of mine.
His life lay glistening in the rain, neglected,
Yet still a decent, an authentic life.
Some people I can think of, I reflected
Would take that thing as soon as you’d say Knife.
It seemed a shame to miss a chance like that.
I brought the life in, dried it by the stove.
It looked so fetching, stretched out on the mat.
I tried it on. It fitted, like a glove.
And now, when some local bat drops off the twig
And new folk take the house, and pull up floors
And knock down walls and hire some kind of big
Container (say, a skip) for their old doors,
I’ll watch it like a hawk, and every day
I’ll make at least – oh – half a dozen trips.
I’ve furnished an existence in that way.
You’d not believe the things you find on skips.