Sharp tender shock
An Arundel Tomb
Coming across it in Chichester cathedral recently, was a shock. My friends had misled me. “It’s not here” they had said. “It’s in Arundel.”
Afterwards, walking outside, a young couple asked for a photo. I told them about the tomb and poem – they didn’t know either – so I recited the final line. They promised to check it out. I started to explain how people misread it, or maybe don’t, but got “a bit o’ grit in me eye”, so had to turn away instead.
An Arundel Tomb
Side by side, their faces blurred,
The earl and countess lie in stone,
Their proper habits vaguely shown
As jointed armour, stiffened pleat,
And that faint hint of the absurd—
The little dogs under their feet.
Such plainness of the pre-baroque
Hardly involves the eye, until
It meets his left-hand gauntlet, still
Clasped empty in the other; and
One sees, with a sharp tender shock,
His hand withdrawn, holding her hand.
They would not think to lie so long.
Such faithfulness in effigy
Was just a detail friends would see:
A sculptor’s sweet commissioned grace
Thrown off in helping to prolong
The Latin names around the base.
They would not guess how early in
Their supine stationary voyage
The air would change to soundless damage,
Turn the old tenantry away;
How soon succeeding eyes begin
To look, not read. Rigidly they
Persisted, linked, through lengths and breadths
Of time. Snow fell, undated. Light
Each summer thronged the glass. A bright
Litter of birdcalls strewed the same
Bone-riddled ground. And up the paths
The endless altered people came,
Washing at their identity.
Now, helpless in the hollow of
An unarmorial age, a trough
Of smoke in slow suspended skeins
Above their scrap of history,
Only an attitude remains:
Time has transfigured them into
Untruth. The stone fidelity
They hardly meant has come to be
Their final blazon, and to prove
Our almost-instinct almost true:
What will survive of us is love.
Philip Larkin
Evie Toombes’ Wrongful Birth
Spina bifida and folic acid
Many were startled last week to learn that Evie Toombes, who has a type of occult spinal lesion, a lipomyelomeningocele, which would not have been prevented by her mother taking folic acid, will be awarded damages against Dr Mitchell, her mother’s GP, who had failed to advise her correctly about taking folic acid. Evie is “an occasional wheelchair/Zimmer frame user”, and needs to self catheterise, but is also “a para-horse rider, a campaigner for hidden disabilities and together with her mother, the author of a book”. Click here for the news story, and here or here for the full judgment.
An earlier court had already ruled on the principle; if, with correct advice, Evie’s mother would have delayed conception, Evie would never have been born and another healthy child replaced her, Evie would be entitled to damages.
There are parallels here with abortion for fetal abnormality. In the 1970’s the Oxford philosopher, Richard Hare, argued that parents considering abortion should decide in the interests of all their potential future children. A mother carrying a fetus with spina bifida should abort in the interests of her, unconceived, but likely to be healthy, replacement child (click here). In my experience this is how many parents think, and I guess the legal arguments follow this sort of logic too. I find the earlier court’s decision rather congenial.
But the judge’s ruling that the doctor’s advice was so bad that no responsible body of GPs would have advised Evie’s mother that way, does seem harsh.
Imagine Dr Mitchell, back when the claim started, getting a lawyer’s letter presumably alleging that he’d failed to advise Evie’s mum to take folic acid. Imagine him pulling his 27 February 2001 note to read “Preconception counselling. adv. Folate if desired discussed” (judgment para 31). He must have felt out of the frame.
But here’s para 61. Both sides agree that Mrs Toombes had attended for preconception counselling at her own instigation.
“I find that Dr Mitchell’s note is completely inadequate. As I have found, Mrs Toombes’ main concern was with regard to stopping the pill and that is not referred to in the note at all. I find that Dr Mitchell’s assumption that “Folate if desired,” means that he gave his usual standard advice, but that Mrs Toombes raised an issue as to whether or not the supplement was necessary and he explored details of her diet, informed her of the risks and then left it to her to choose, is nothing but speculation after the event. The note gives the impression without more discussion that Mrs Toombes was told that she should take folic acid if she wanted to. I accept Mrs Toombes’ evidence that she came away from the consultation under the impression that if she had a healthy diet, folic acid supplements were not necessary. In the circumstances, I prefer the evidence of Mrs Toombes and find that she was not told about the recommended dose or the reason why folic acid supplementation was recommended or that it should be taken before conception and for the first 12 weeks of pregnancy.”
Nothing wrong with the judge making findings of fact. But we can now see the aspects of Dr Mitchell’s advice, that were so bad that apparently no responsible body of GPs would follow them. Here they are.
- Qualifying his advice with a comment that folic acid was not necessary if her diet was good
- Not telling her the exact dose
- Not saying that the reason was to prevent spina bifida
- Not saying that it should be given pre-conception
To a woman who was knowledgable enough to arrange preconception counselling! We used to think obstetricians had it tough.
Jim Thornton
Gynaecological effects of Covid
Scientific papers reporting short and long-term effects of Covid-19 on menstruation, menarche and menopause, fertility, genital tumours, uterovaginal prolapse, female sexual function, urinary and vulval disorders, endometriosis and other miscellaneous gynaecological problems. Primary sources only. Updated regularly. Most recent first. Curated by Jim Thornton, Keelin O’Donoghue & Kate Walker.
For Covid-19 in pregnancy (click here), indirect effects on pregnancy (click here), news reports (click here) and effects of Covid-19 vaccines (click here).
8 March – GP 25 added
Gynae paper 25 – no effect of vaccination in Israel
From the IVF units, Shamir, Sheba, and Herzliya Medical Centres, Tel Aviv (click here or paper). COVID-19 mRNA vaccine did not affect the ovarian response or pregnancy rates. Citation: Avraham S, Kedem A, Zur H, Youngster M, Yaakov O, Yerushalmi GM, Gat I, Gidoni Y, Hochberg A, Baum M, Hourvitz A, Maman E, COVID-19 Vaccination and Infertility Treatment Outcomes, Fertility and Sterility (2022), doi: https://doi.org/10.1016/j.fertnstert.2022.02.025.
20 February – GP 24 added
Gynae paper 24 – case of ovarian vein thrombosis in USA
From Reading Hospital Tower Health, Reading (click here or study). Citation: DeBoer RE, Oladunjoye OO, Herb R. Right Ovarian Vein Thrombosis in the Setting of COVID-19 Infection. Cureus. 2021 Jan 20;13(1):e12796. doi: 10.7759/cureus.12796. PMID: 33628665; PMCID: PMC7893676.
6 February – GP 23 added
Gynae paper 23 – indirect effect of the pandemic on menstruation
1159 women from Andorra, Australia, Canada, Colombia, Estonia, Germany, Israel, Latvia, Netherlands, Romania, Spain, Sweden, Switzerland, UK, and USA, using the Daysy fertility device from 1 January to 30 June, in both 2019 and 2020 (click here or paper). Citation: Haile L, van de Roemer N, Gemzell-Danielsson K, Perelló Capó J, Lete Lasa I, Vannuccini S, Koch MC, Hildebrandt T, Calaf J. The global pandemic and changes in women’s reproductive health: an observational study. Eur J Contracept Reprod Health Care. 2022 Jan 18:1-5. doi: 10.1080/13625187.2021.2024161. Epub ahead of print. PMID: 35040737.
26 January 2022 – GP 16 to 22 added. GP 13 transferred to vaccines & fertility (click here)
Gynae paper 22 – vaccine response in 36 women with ovarian cancer
Centres not reported, but approved by the research ethics committee of Alexandra Hospital, Athens, Greece (click here or paper). Citation: Liontos, M.; Terpos, E.; Markellos, C.; Zagouri, F.; Briasoulis, A.; Katsiana, I.; Skafida, E.; Fiste, O.; Kunadis, E.; Andrikopoulou, A.; Kaparelou, M.; Koutsoukos, K.; Gavriatopoulou, M.; Kastritis, E.; Trougakos, I.P.; Dimopoulos, M.-A. Immunological Response to COVID-19 Vaccination in Ovarian Cancer Patients Receiving PARP Inhibitors. Vaccines 2021, 9, 1148. https://doi.org/10.3390/vaccines9101148
Gynae paper 21 – 46 cases from Argentina
Women, aged 21–41, undergoing assisted reproductive technology procedures between November 2020 and April 2021 at the following clinics; PREGNA Medicina Reproductiva, IVI Buenos Aires, Fertilis, and InVitro, all in Buenos Aires (click here or paper). Citation: Herrero Y, Pascuali N, Velázquez C, Oubiña G, Hauk V, de Zúñiga I, Peña MG, Martínez G, Lavolpe M, Veiga F, Neuspiller F, Abramovich D, Scotti L, Parborell F. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim Biophys Acta Mol Basis Dis. 2022 Jan 1;1868(1):166295. doi: 10.1016/j.bbadis.2021.166295. Epub 2021 Oct 27. PMID: 34718118; PMCID: PMC8550892. – Control study
Gynae paper 20 – 9 couples from Israel
Seven with female partner covid, and two with male partner, teated at Chaim Sheba Medical Center, Ramat Gan, both before the pandemic and after infection (click here or paper). Citation: Orvieto R, Segev-Zahav A, Aizer A. Does COVID-19 infection influence patients’ performance during IVF-ET cycle?: an observational study. Gynecol Endocrinol. 2021 Oct;37(10):895-897. doi: 10.1080/09513590.2021.1918080. Epub 2021 May 11. PMID: 33974475.
Gynae paper 19 – case report from Turkey
From author affiliations the Atasehir Memorial IVF Center, Istanbul (click here or paper). Citation: Demirel C, Tulek F, Celik HG, Donmez E, Tuysuz G, Gökcan B. Failure to Detect Viral RNA in Follicular Fluid Aspirates from a SARS-CoV-2-Positive Woman. Reprod Sci. 2021 Aug;28(8):2144-2146. doi: 10.1007/s43032-021-00502-9. Epub 2021 Feb 22. PMID: 33616884; PMCID: PMC7899067.
Gynae paper 18 – 2 case reports from Spain
From author affiliations the Clinica EUGIN, Carrer de Balmes 236, Barcelona (click here or paper). Citation: Barragan M, Guillén JJ, Martin-Palomino N, Rodriguez A, Vassena R. Undetectable viral RNA in oocytes from SARS-CoV-2 positive women. Hum Reprod. 2021 Jan 25;36(2):390-394. doi: 10.1093/humrep/deaa284. PMID: 32998162; PMCID: PMC7543480.
Gynae paper 18 – 9 cases from Israel
See vaccination source 23 here.
Gynae paper 17 – 14 cases from France
From the ART unit of Tenon Hospital, Paris, between June and December 2020 (click here or paper). Citation: Kolanska K, Hours A, Jonquière L, Mathieu d’Argent E, Dabi Y, Dupont C, Touboul C, Antoine JM, Chabbert-Buffet N, Daraï E. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod Biomed Online. 2021 Dec;43(6):1117-1121. doi: 10.1016/j.rbmo.2021.09.001. Epub 2021 Sep 10. PMID: 34711516; PMCID: PMC8432972.
Gynae paper 16 – 70 cases from China
From the Reproductive Medicine Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between May 2020 and February 2021 (click here or paper). Citation: Wang M, Yang Q, Ren X, Hu J, Li Z, Long R, Xi Q, Zhu L, Jin L. Investigating the impact of asymptomatic or mild SARS-CoV-2 infection on female fertility and in vitro fertilization outcomes: A retrospective cohort study. EClinicalMedicine. 2021 Aug;38:101013. doi: 10.1016/j.eclinm.2021.101013. Epub 2021 Jul 6. PMID: 34250457; PMCID: PMC8259363.
25 January 2022 – GP 15 added
Gynae paper 15 – 78 cases from China
Women of reproductive age with Covid between January 28 and March 8, 2020 from Tongji Hospital in Wuhan (click here or paper). Citation: Ding T, Wang T, Zhang J, Cui P, Chen Z, Zhou S, Yuan S, Ma W, Zhang M, Rong Y, Chang J, Miao X, Ma X, Wang S. Analysis of Ovarian Injury Associated With COVID-19 Disease in Reproductive-Aged Women in Wuhan, China: An Observational Study. Front Med (Lausanne). 2021 Mar 19;8:635255. doi: 10.3389/fmed.2021.635255. PMID: 33816526; PMCID: PMC8017139.
7 January 2022 – GP 14 added
Gynae paper 14 – vaccination & menstrual cycle length
US resident participants enrolled in the fertility-awareness application “Natural Cycles”, who recorded menstrual cycle data from October 2020 to September 2021, and were vaccinated between December 2020 and July 2021, and an unvaccinated control group (click here or paper). Citation: Edelman, Alison MD, MPH; Boniface, Emily R. MPH; Benhar, Eleonora PhD; Han, Leo MD, MPH; Matteson, Kristen A. MD, MPH; Favaro, Carlotta PhD; Pearson, Jack T. PhD; Darney, Blair G. PhD, MPH Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination, Obstetrics & Gynecology: January 5, 2022 – Volume – Issue – 10.1097/AOG.0000000000004695 doi: 10.1097/AOG.0000000000004695
23 December – GP 13 added
Gynae paper 13 – no effect of mRNA vaccine on AMH levels
129 women given two doses of the Pfizer-BioNTech vaccine (click here or paper). Citation: Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Amdurski HD, Dadon T, Blumenfeld S, Derazne E, Hemi R, Orvieto R, Afek A, Rabinovici J. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod. 2021 Dec 22:deab282. doi: 10.1093/humrep/deab282. Epub ahead of print. PMID: 34935913. 26 January update – transferred to Covid-19 vaccines, pregnancy & fertility, source 95 (click here).
13 December – GP 12 added
Gynae paper 12 – 8,538 vaccinated women
Volunteers participating in The Covid-19 Pandemic and Women’s Reproductive Health registry (click here or paper). Citation: Alexandra Alvergne, Gabriella Kountourides, Austin Argentieri, Lisa Agyen, Natalie Rogers, Dawn Knight, Gemma C Sharp, Jacqueline A Maybin , Zuzanna Olszewska. COVID-19 vaccination and menstrual cycle changes: A United Kingdom (UK) retrospective case-control study. MedRxiv posted December 6, 2021
30 November – GP 10 and 11 added
Gynae paper 11 – RCT of estadiol Rx in post menopausal women with Covid in India
Recruited at the Government Institute of Medical Sciences, Greater Noida, from September 10 to December 31, 2020 (click here or paper). Citation: Seth S, Sharma R, Mishra P, Solanki HK, Singh M, Singh M. Role of Short-Term Estradiol Supplementation in Symptomatic Postmenopausal COVID-19 Females: A Randomized Controlled Trial. J Midlife Health. 2021 Jul-Sep;12(3):211-218. doi: 10.4103/jmh.JMH_57_21. Epub 2021 Oct 16. PMID: 34759703; PMCID: PMC8569453.
Gynae paper 10 – 14 cases undergoing ART in France
From Tenon Hospital, Paris (click here or paper). Citation: Kolanska K, Hours A, Jonquière L, Mathieu d’Argent E, Dabi Y, Dupont C, Touboul C, Antoine JM, Chabbert-Buffet N, Daraï E. Mild COVID-19 infection does not alter the ovarian reserve in women treated with ART. Reprod Biomed Online. 2021 Sep 10:S1472-6483(21)00431-4. doi: 10.1016/j.rbmo.2021.09.001. Epub ahead of print. PMID: 34711516; PMCID: PMC8432972.
18 November – GP 8 and 9 added
Gynae paper 9 – case report (ectopic & Covid) from USA
From University of Miami, Health Systems, Jackson Memorial Hospital, Miami, Florida (click here or paper). Citation: Millan NM, Morano J, Florez L, Carugno J, Medina CA. Management of tubal ectopic pregnancy with methotrexate in the setting of symptomatic Coronavirus disease 2019 (COVID-19): A case report. Facts Views Vis Obgyn. 2021 Sep;13(3):277-281. doi: 10.52054/FVVO.13.3.030. PMID: 34555882.
Gynae paper 8 – Case report (ectopic & Covid) from Japan
From Gifu Prefectural Tajimi Hospital (click here or paper). Citation: Hayashi S, Takeda A. Gasless laparoendoscopic single-site surgery for management of unruptured tubal pregnancy in a woman with moderate COVID-19 pneumonia after administration of remdesivir and casirivimab-imdevimab: A case report. Case Rep Womens Health. 2022 Jan;33:e00368. doi: 10.1016/j.crwh.2021.e00368. Epub 2021 Nov 11. PMID: 34786352; PMCID: PMC8580554.
9 November – GP 7 added
Gynae paper 7 – Endometriosis survey from Italy
468 women diagnosed with endometriosis at Dipartimento di Scienze Mediche e Chirurgiche, Sant’Orsola-Malpighi Hospital, University of Bologna, and surveyed in May 2020 (click here or paper). Citation: Arena A, Orsini B, Degli Esposti E, Raimondo D, Lenzi J, Verrelli L, Iodice R, Casadio P, Seracchioli R. Effects of the SARS-CoV-2 pandemic on women affected by endometriosis: a large cross-sectional online survey. Ann Med. 2021 Dec;53(1):1924-1934. doi: 10.1080/07853890.2021.1991589. PMID: 34714186; PMCID: PMC8567944.
1 November 2021
Gynae paper 6 – 507 women with, & 520 without, endometriosis in Iran
No difference in rates or severity of Covid (click here or paper). A case–control study at Pars general hospital, Tehran, from May 21st to July 3rd, 2020. Citation: Moazzami B, Chaichian S, Samie S, Zolbin MM, Jesmi F, Akhlaghdoust M, Pishkuhi MA, Mirshafiei ZS, Khalilzadeh F, Safari D. Does endometriosis increase susceptibility to COVID-19 infections? A case-control study in women of reproductive age. BMC Womens Health. 2021 Mar 22;21(1):119. doi: 10.1186/s12905-021-01270-z. PMID: 33752656; PMCID: PMC7983080.
Gynae paper 5 – Haemothorax after IVF & Covid
From Conceber Centro de Medicina Reprodutiva, Curitiba, Paraná, Brazil (click here or paper). Citation: Rahal D, Kozlowski IF, Rosa VBD, Schuffner A. Hemothorax after oocyte retrieval in a patient with a history of COVID-19: a case report. JBRA Assist Reprod. 2021 Oct 4;25(4):647-649. doi: 10.5935/1518-0557.20210043. PMID: 34415132; PMCID: PMC8489828.
Gynae paper 4 – Covid, menopause & hormone therapy
Covid was more common among postmenopausal women and, within the latter group, more common among those taking menopausal hormone therapy (click here or paper). Women using the “Zoe” Covid Symptom Study app. Citation: Costeira R, Lee KA, Murray B, Christiansen C, Castillo-Fernandez J, Ni Lochlainn M, et al. (2021) Estrogen and COVID-19 symptoms: Associations in women from the COVID Symptom Study. PLoS ONE 16(9): e0257051. https://doi.org/10.1371/journal.pone.0257051
Gynae paper 3 – 237 women with Covid from China
Women aged 18 to 45 with confirmed COVID-19 who were hospitalized in Tongji Hospital, Wuhan, from 19 January to 1 April 2020 (click here or paper). Citation: Li K, Chen G, Hou H, Liao Q, Chen J, Bai H, Lee S, Wang C, Li H, Cheng L, Ai J. Analysis of sex hormones and menstruation in COVID-19 women of child-bearing age. Reprod Biomed Online. 2021 Jan;42(1):260-267. doi: 10.1016/j.rbmo.2020.09.020. Epub 2020 Sep 29. PMID: 33288478; PMCID: PMC7522626.
Gynae paper 2 – Mobile app ovulation & menstruation
No overall changes before and during pandemic in 214,426 cycles from 18,076 app users, primarily from Great Britain (29.3%) and the United States (22.6%) (click here or paper). Women using the “Natural Cycles” mobile app. Citation: Nguyen BT, Pang RD, Nelson AL, Pearson JT, Benhar Noccioli E, Reissner HR, Kraker von Schwarzenfeld A, Acuna J. Detecting variations in ovulation and menstruation during the COVID-19 pandemic, using real-world mobile app data. PLoS One. 2021 Oct 20;16(10):e0258314. doi: 10.1371/journal.pone.0258314. PMID: 34669726; PMCID: PMC8528316.
Gynae paper 1 – Menstrual symptoms in 127 self-selected women with Covid
From the Arizona CoVHORT study (click here or paper). Citation: Khan SM, Shilen A, Heslin KM, Ishimwe P, Allen AM, Jacobs ET, Farland LV. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT Study. Am J Obstet Gynecol. 2021 Sep 20:S0002-9378(21)01044-9. doi: 10.1016/j.ajog.2021.09.016. Epub ahead of print. PMID: 34555320; PMCID: PMC8452349.
Jim Thornton, Keelin O’Donoghue & Kate Walker
My first PubPeer comment
As anyone who’s tried to expose fraudulent research by writing a “letter to the editor” will know, it can be fustrating. Editors prevaricate, authors obfuscate, and time ticks by.
PubPeer (click here) is an alternative. Publish the facts, let the author reply, and readers, editors, regulators and employers can judge for themselves. If you enter the author’s email when you submit, PubPeer automatically notifies them that one of their papers has been commented on.
It has been a powerful way to expose fabricated laboratory research, where authors have manipulated images. It can also be used for fabricated clinical studies.
To protect whistleblowers, comments can be anonymous – they just need to stick to verifiable facts – but I’ll be signing mine. Here’s my first. To read it on PubPeer (click here).
It refers to this paper:
Rezk M, Shaheen AE, Saif El-Nasr I. Clomiphene citrate combined with metformin versus letrozole for induction of ovulation in clomiphene-resistant polycystic ovary syndrome: a randomized clinical trial. Gynecol Endocrinol. 2018 Apr;34(4):298-300. doi: 10.1080/09513590.2017.1395838. Epub 2017 Oct 27. PMID: 29076376.
Table 1 has identical values for FSH and LH as Table 1 in a different paper in the same journal from 2016.
Rezk M, Sayyed T, Saleh S. Impact of unilateral versus bilateral laparoscopic ovarian drilling on ovarian reserve and pregnancy rate: a randomized clinical trial. Gynecol Endocrinol. 2016;32(5):399-402. doi: 10.3109/09513590.2015.1124262. Epub 2015 Dec 15. PMID: 26670076.
Present paper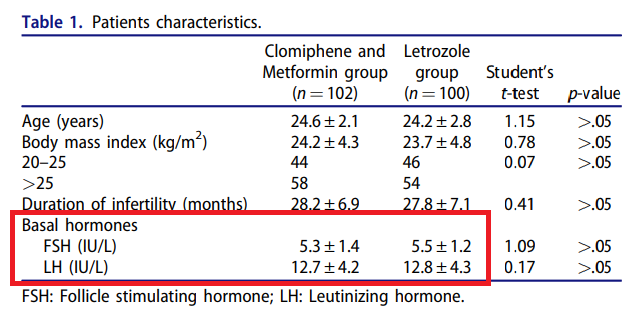
Rezk et al 2016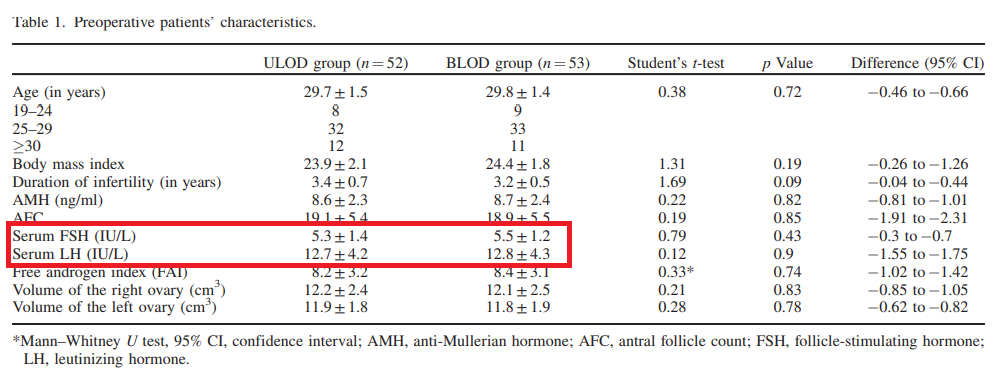
The earlier paper reported a trial comparing two surgical treatments with 105 participants, and the present paper a trial of two medical treatments with 202 participants. The earlier paper recruited “from October 2014 to July 2015”. The present paper recruited “between the middle of October 2016 and the beginning of May 2017”. The later paper does not reference the earlier one.
Both have the same first author, and originate from Menoufia university, Egypt.
Jim Thornton
Why use ripe-tomato as a Covid in pregnancy source?
People often ask us “What’s different about ripe-tomato? Why not use WHO (click here), Cochrane (click here), or PubMed (click here) Covid-19 databases instead?”
Serious systematic reviewers, or guideline authors, should indeed use all those. But it will be a lot of work, searching, accessing, reading and deduplicating.
Ripe-tomato has done it for you, for primary sources in pregnancy. It isn’t perfect, but we’ve not missed much. Friends and colleagues soon tell us if we have!
But haven’t the WHO/Cochrane Living Systematic Review (LSR) team and Johns Hopkins each done the work too? Yes and no.
Shakila Thangiratinam and her WHO/Cochrane LSR team (click here) have a more complete, and better indexed, database. Dozens of people update it full time – and check ripe-tomato. But it’s not publicly accessible. The reference lists and appendices of their publications reveal the sources they used, but they keep the master database to themselves.
Johns Hopkins Center for Humanitarian Health has two related repositories. COVID-19, Maternal and Child Health, and Nutrition (click here) and COVID-19, Breastfeeding, Infant Feeding, and Breast Milk (click here). Both broader in topic than ripe-tomato and not limited to primary sources. Update 1 May 2021. The two John Hopkins repositories ceased updates from 30 April. They remain publicly available but are no longer updated.
Ripe-tomato’s unique features
Primary sources only. Anyone whose ever studied Covid-19 realises that secondary sources, reviews, editorials, comments and opinion pieces outnumber the primary sources many times. Not on ripe-tomato.org
Duplicate publications noted. Another major problem for all Covid evidence synthesisers. If you’re writing a review, creating a reference list for an article, or refereeing a paper on Covid-19 in pregnancy, ripe-tomato is your friend. We’ve caught and recorded most of the obvious duplicates. Use us.
Easy access. Some sources are behind paywalls, and some difficult to access. Don’t ask how we do it. But where possible, beside a link to the source website, we also provide a direct link to our own uploaded copy.
Newspaper reports (click here). A deduplicated list of primary newspaper stories about people with Covid-19 in pregnancy. From Italy’s “patient one”, the partner of Juventus footballer, Daniele Rugani, and the wife of the British Prime Minster, to a transgender man who caught Covid during his pregnancy. The list of nearly 700 heartbeaking and heartwarming stories is unique. It’s not complete – English language reports are over represented – but there’s nothing else like it in the world.
Click here for Ripe-tomato.org’s Covid-19 in pregnancy navigation page.
Jim Thornton
Bloody awful tripe?
This Be The Verse and The Trees
This Be The Verse is Philip Larkin’s most famous poem. In his latest book, Inside Story, (p 117) Martin Amis notes that it has a technically near identical sister, The Trees, which is more optimistic. Although Larkin apparently wrote “Bloody awful tripe” on the manuscript of the latter, he published it in High Windows, and it’s not.
This Be the Verse
They fuck you up, your mum and dad.
They may not mean to, but they do.
They fill you with the faults they had
And add some extra, just for you.
But they were fucked up in their turn
By fools in old-style hats and coats,
Who half the time were soppy-stern
And half at one another’s throats.
Man hands on misery to man.
It deepens like a coastal shelf.
Get out as early as you can,
And don’t have any kids yourself.
The Trees
The trees are coming into leaf
Like something almost being said;
The recent buds relax and spread,
Their greenness is a kind of grief.
Is it that they are born again
And we grow old? No, they die too.
Their yearly trick of looking new
Is written down in rings of grain.
Yet still the unresting castles thresh
In fullgrown thickness every May.
Last year is dead, they seem to say,
Begin afresh, afresh, afresh.
(Not) Giving Women Good Sex
Is this fraud?
My colleague, Susan Bewley, drew my attention to the Journal of Sex Research last week (click here or Spielmans 2020 Re Analyzing Phase III Bremelanotide Trials for Hypoactive Sexual Desire Disorder in Women). The author, Glen Spielmans, Professor of Psychology at Metropolitan State University in Minnesota, dissects two pivotal phase-3 trials (click here or Kingsberg Obstetrics & Gynecology November 2019), which had led the US Federal Drug Administration (FDA) to license bremelanotide, trade name Vyleesi, to treat women with hypoactive sexual desire disorder (HSDD).
One of the original co-primary outcomes in each trial was switched, many pre-specified outcomes went unreported and new ones appeared, scores were reported as dichotomous rather than as the prespecified means, cut-off values varied for no apparent reason, and other outcomes were claimed as supportive without data. Spielmans also noted that more women (18 or 19% bremelanotide v 3 or 9% placebo) dropped out of the active groups, and questioned whether the various shifts in subsets of sexual behaviour scores, even in the unlikely event they were real, were of clinical significance.
He is correct on all counts. But altered co-primary outcomes in two drug licensing trials? How did that get past the FDA?
Background
Bremelanotide is a polypeptide, related to melanocyte stimulating hormone (MSH). Ever since someone noticed in the 1960s that MSH caused rats to become sexually aroused, chemists have been fiddling with the pharmacology in the hope of creating a female Viagra. In 2016, Palatin Technologies, who own the rights, convinced themselves in a phase-2 dose-finding trial (PT-141-54 in the FDA report) (click here or clayton 2016) that the stuff worked at higher doses. The planned sample size was 100 per group. The pre-specified primary outcome was change in satisfying sexual events (SSE) per month.
| Placebo | Low dose | Medium | High dose | |
| No. randomised | 99 | 100 | 99 | 99 |
| Analysed (at least 1 dose & 1 follow-up visit) | 91 | 87 | 75 | 74 |
| Dropped out | 8 | 13 | 24 | 25 |
| Additional satisfying sexual events per month | 0.2 | 0.6 | 0.7 | 0.8 |
The authors claimed a dose response relationship (bottom row) but were coy about the row above – I’ve extracted it for you. Without knowing what they were getting, three times as many women on the higher doses dropped out. In a double-blind trial this can only mean one thing, side effects. There were many more, mainly nausea, flushing and headache, in the active treatment groups
Such non-random differential drop out pretty much invalidates the results. If women who stayed the course were more stoical, or had a greater desire for their sexual problems to be sorted, it’s hardly surprising to see a small increase in SSEs.
But what do I know? These data were judged sufficiently promising to set up two pivotal efficacy trials for FDA approval.
The trials
Both had the same title “Study to Evaluate the Efficacy/Safety of Bremelanotide in Premenopausal Women With Hypoactive Sexual Desire Disorder (HSSD)” on Clinicaltrials.gov.
They both studied the allegedly most effective, high dose of bremelanotide, 1.75mg, from the dose-finding study.
They were both reported in the same paper (click here or Kingsberg Obstetrics & Gynecology November 2019). For the FDA analysis (click here or FDA bremelanotide).
Study 1 (labelled BMT-301 in Kingsberg and FDA papers) had a planned sample size of 550 (actual 723) and ran from December 2014 to July 2016, (registry here). Study 2 (labelled BMT-302) had the same planned sample size of 550 (actual 714) and ran from January 2015 to August 2016, (registry here). I could find no explanation for the 30% sample size increases, in either the Kingsberg paper or the FDA report.
Despite their identical eligibility criteria, and almost identical recruitment periods, both trials appear to have been run in the same 91 centres.
The original co-primaries for both trials were:
1. Change in Female Sexual Function Index–desire domain (FSFI-D). The desire domain is the sum of; “Over the past 4 weeks, how often did you feel sexual desire or interest?” – responses range from 1 “Almost never or never” to 5 “Almost always or always” – and; “Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?” – responses range from 1 “Very low or none at all” to 5 “Very high”.
2. Change in absolute number of sexually satisfying events (SSE) per month.
Of thirteen planned secondary outcomes, the twelfth was this question from the Female Sexual Distress Scale–Desire/Arousal/Orgasm (FSDS-DAO); “are you bothered by low sexual desire?” Responses range from 0 “never” to 4 “always”.
Both trial results would have been negative had the co-primary outcomes remained unchanged. Although absolute numbers of SSEs are not reported in either the paper nor the FDA report, the latter states “neither treatment nor placebo arm showed any improvement” (p 126).
This looks suspicious. SSEs are more meaningful than “bothered by low sexual desire” which is little more than a different aspect of the other co-primary.
But on 27 October 2014 (click here) the FDA had held a workshop with patients about Female Sexual Dysfunction.
The company claimed that workshop participants had stated that SSEs were a poor efficacy outcome. Some quotations e.g. “Several [participants] stressed the importance of feeling desire regardless of whether it is accompanied by a satisfying sexual event” (p6), could be interpreted this way, although others e.g. “Participants largely appeared to believe that having satisfying sexual events was important to them” (p13) suggest the opposite. Even on its own terms this is hardly a convincing argument.
But the workshop had been captured by pharma. Here’s what independent experts, Leonore Tiefer, Ellen Laan and Rosemary Basson wrote about it at the time (click here or Tiefer),
Participants had their expenses paid by [a group funded by] pharmaceutical companies, […] had met the morning of the first day in their hotel to hear presentations and prepare their talking points.
They had each received a green shawl, identifying them with the ‘’even the score’’ campaign that accused the FDA of sexism in handling [female sexual disorder] drug applications [and] arrived at and departed together by chartered bus.
It looks like the FDA was pressured into agreeing the switch, to avoid accusations of sexism in the way they treated applications for female and male sexual treatments.
And the timing of the switch? The alteration was requested on 10 October 2016. Study BMT-301 had been completed on July 26 that year, and study BMT-302 on Aug 4th.
Let’s summarise. More than two months after the studies had been completed, on the basis of a dodgy, pharma-captured workshop conducted two years earlier, the trial sponsors persuaded the FDA to allow the co-primary endpoints to be switched. They were changed from a meaningful predefined outcome justified by the pilot dose-finding study, the number of satisfying sexual events, to the twelfth of thirteen secondary outcomes. And guess what? Bremelanotide, which did not alter the number of satisfying sexual events, magically improved this suddenly important secondary outcome.
Spielmans doesn’t say it, but I do. This must be a data-driven outcome switch. If so, that’s fraud.
Jim Thornton
Update 10 April 2021
Yesterday Dr Kingsberg and her colleagues replied to Dr Spielmans’ critique (click here or paper), and Dr Spielmans replied to her reply (click here or paper). Despite much bluster, in my opinion, she failed to deal with any of his substantive points. But judge for yourself.
With regard to whether the co-primary outcome switch was data driven, Kingsberg writes;
He also alludes to “data peeking” in his introduction and that the FDA allowed the “sponsor’s request for satisfying sexual events (SSEs) to move from the co-primary to the key secondary outcome …. a year after the trials had begun.” What Spielmans omitted is that the FDA published a guidance document (2016) for designing clinical trials in which SSEs were no longer required to be a primary endpoint for HSDD treatment trials. Instead, trials could now include measures reflecting the hallmark criteria of the condition: loss of (i.e., deficiency or absence of) sexual desire (i.e., FSFI-D) and distress about lack of desire (i.e., FSDS-DAO #13). The approval from the FDA to change the primary endpoint, after discussion with the FDA review division, came prior to the data lock [my emphasis] in these well-conducted, randomized, double-blind, multicenter placebo-controlled trials, with pre-established statistical analysis plans.
Note the non-denial choice of words. No-one doubts that the outcome switch came “prior to the data lock”. The issue is whether the sponsor had seen the data prior to the outcome switch request.
Philosophy for children P4C2 – clarification
The power of randomisation
Mistake in my P4C-2 post yesterday (click here). The result is still negative, and the report still turgid, but when describing the distribution of the pooled reading and maths scores, I suggested that the authors had not shown them by group, and made the snide comment “Perish the thought that an educationalist would ever show you anything remotely near to the raw data!”
Forgive me. Further down appendix I of the main report (click here) are “intervention” (p 100) and, controls (p 102). But different pages? This is a randomised trial where half the pupils got philosophy teaching and half did not. Readers want to compare the two groups side by side. Come on EEF!
To help I’ve reordered the graphs here p4c2 result histograms p number. Intervention group left, control right. Page one, free school meal (FSM) pupils and page two all pupils. Reading scores before maths scores.
It’s rather revealing. For each outcome and for each subgroup not only are the means and ranges almost identical but also the shapes of the distributions. Not exactly identical – these are different populations – but remarkably similar. Go on, check. Click the link above and then scroll down comparing left to right. The shape of the control distribution of reading scores among FSM pupils is different from the shape of the control maths scores, but both are almost identical to the respective reading and maths scores for the intervention group. Same for the whole sample.
Not only did the intervention, Philosophy for Children, have no effect on maths or reading but, since it had no effect, we can see how beautifully, when you have a large sample size, randomisation really does generate comparable groups.
Well I think it beautiful.
Jim Thornton
Philosophy for Children (P4C) – trial 2
Still doesn’t work. Really doesn’t. Not even a tiny benefit.
Five years ago a rather nice randomised trial, run by the Education Endowment Foundation (EEF) (click here), tested the effect on reading and writing, of teaching philosophy to primary school children. The result was negative, but unwisely the EEF entrusted evaluation to a group of educationalists who were determined to find a positive result. By ignoring their original analysis plan, doing a data-driven analysis of change scores, and picking a favourable subgroup, children entitled to free school meals, they managed to convince themselves that teaching little children to be kind to their teddy bears helped wth reading and maths (click here for my analysis of where they went wrong). The newspapers picked up the story and headlines flew round the world (click here).
Fortunately I wasn’t the only one to smell a rat (see here and here). Professor Gorard, the lead evaluator, sent me a patronising email and blocked me on social media, but the EEF did the right thing. They realised they’d boobed, decided not to published the misleading analysis in a peer-reviewed journal [but see footnote], and did the trial again. The results are just in (website here, main report here, or for those with access problems Philosophy_for_Children_report_-_final_-_pdf).
The second trial, P4C-2, was also a cluster design but larger, 75 intervention and 123 control schools, compared with the original trial’s 26 intervention and 22 control. The protocol (click here) and analysis plan (click here) were published and adhered to. The biggest risk with cluster trials is differential recruitment or measurement of outcomes related to knowing the cluster, but this was avoided by using the 2019 Key Stage 2 (KS2) reading scale score as the primary outcome. KS2 reading and maths are measured independently on all pupils in the country, whether or not they or their schools participated. Since Gorard’s data dredging had shown the most “benefit” among children receving free school meals, the primary analysis was planned for this group. Analysis was by intention to treat.
The report is 125 pages long, and a turgid read, – education researchers love making simple things complicated – but the essence is easy to describe. There is a CONSORT flow diagram (Fig 1, p33 not shown here), few pupils were lost to follow-up (Table 12, p36 not shown here), and the randomisation achieved a balance of both schools and pupils at baseline (Table 13, p37 not shown here). The primary and main secondary outcomes are in Table 14, p38 (click on thumbnail to read).
The presentation is rather strange. For each group they report the mean score and the 95% confidence interval for the mean. The actual score distributions are relegated to appendix I, p98.
Don’t be mislead by the words intervention and control into reading these as distributions by group. Perish the thought that an educationalist would ever show you anything remotely near to the raw data! These are pooled results for reading and maths, each for the whole sample and the free school meals subgroup; four distributions in total. They tell us nothing about the effect of the intervention, but they allow the reader to see that scores are roughly normally distributed with mean values between 100 and 105, a range of 80-120 and a standard deviation of roughly 12.
Turning back to table 14 above we can see that the absolute mean differences between group are tiny fractions of a single score point, i.e trivial. The Hedges g column expresses the difference as a fraction of the pooled standard deviation. Tiny by any standards. Even those educationalists who prefer the effect size to significance testing, ignore effect sizes of <0.2 and generally only consider one of >0.4 as “meaningful”. These are 0.01 to 0.05. For frequentists like me, despite the large sample size, the result is not remotely “statistically significant”.
The rest of the report discusses how well various schools implemented the intervention and searches for a signal related to how well P4C was implemented, but finds nothing.
In summary a well-designed and well-analysed negative trial. We can be confident of the result, and confident also that the trial has not missed a worthwhile small effect.
Some Twitter commenters have said, “But surely we don’t teach moral philosophy to help chidren with reading or maths. We do it to help them grow up to be better kinder people.” They make a good point. The trial says nothing about the effect of P4C on moral behaviour, and everyone supports primary school teachers continuing to teach their pupils to be kind and honest. But education planners should stop paying large sums to Sapere, the creator of P4C, and displacing lessons to teach it, in the hope of improving reading and maths.
It’s a ground-breaking trial
I wonder where it will get published. In my field a negative, large well-conducted cluster trial like this, – the AFFIRM trial of encouraging awareness of fetal movements in pregnancy for example – doesn’t languish in an obscure specialist journal but gets published as a full length paper in the most prestigious medical journal in the world, The Lancet (click here). This report will need a rewrite, but a major general science journal, Science or Nature, should publish it for its methodological importance.
The EEF has shown how to evaluate an educational intervention properly. Imagine if they used the same methods to compare phonics with whole language to teach reading, or to test whether drilling children in tables was helpful or harmful. Imagine that!
Jim Thornton
Footnote added 13 March. I’d missed it. The EEF did publish the misleading analysis in a peer reviewed journal, (click here or 1467-9752.12227), albeit with weaker conclusions. “… for […] attainment outcomes in the short term, an emphasis on developing reasoning is promising, especially for the poorest students, but perhaps not the most effective way forward.”
The singer, the painter & the randy politician
Ann Ford by Thomas Gainsborough
It’s 1760. Bath is booming. The agricultural revolution has increased the population, and created a new class of landed gentry, but the industrial revolution has hardly started. Coal is mined locally, the town is dirty and smoky, and there are no canals, let alone railways. Just stage coaches along the fast but dangerous new turnpikes, often past the bodies of hanged highwaymen. But Bath is fashionable. Wealthy gentry move their households to the first English spa town for the winter season. Gainsborough, facing stiff competition in London, decides to try his hand. This is his advertisement.
21-year-old Ann Ford had started singing privately at her father’s house in London, but has had to run away to Bath to sing in public. She is good, but the crowds titter at a woman behaving so outrageously. Philip Thicknesse, Gainsborough’s mentor, and later Ann’s husband, sets up a meeting, and soon the six foot painting stands in the front window of Gainsborough’s studio. The silvery dress attracts attention and the pose is exciting. No well brought up woman crosses her legs in public! As one future customer notes “a most extraordinary figure, handsome and bold; but I should be very sorry to have any one I loved set forth in that manner.” The viola da gamba, a man’s instrument, peeping out from behind the red curtain, refers to the latest scandal .
William Villiers, the 52-year-old Earl of Jersey, wants Ann, and offers her the extraordinary sum of £800 a year to be his mistress, with the promise of marriage when his ailing wife dies. Not only does she refuse, but when he tries to sabotage her first public concert in retaliation, she publishes a pamphlet, A Letter from Miss F—d to a Person of Distinction, defending her position. Amazingly Villiers replies, and a juicy pamphlet war ensues – the Kim Kardashian of Bath scrapping with a publicity hungry politician. Fun for all, and great for Gainsborough. His career thrives.
The painting is now in the Cincinnati Art Museum (click here).
Jim Thornton
Above mostly from Gainsbrough; a portrait by James Hamilton. Weidenfield & Nicholson, London 2017.



